5 New Covid Vaccine Studies, Nektar NKTR breaks out
Welcome new subscribers. I am still very small here so please help by sharing and subscribing while this is still free. Numbers and analyses to plain English is part of my game.
There is a pile of new information coming out on the Covid Vaccines and countless lawsuits that are seeing positive momentum against the Covid authorities. However, I am just sticking to an update every month or two on the most important new information. It is important we learn from this and be vigilant because they seemed determined to keep pushing out this very profitable mRNA based technology, regardless of safety. It's probably going to be in next years influenza flu shot. I don't think they can stop because they are stuck with their previous lies and can't admit otherwise. And they make way too much $$
Five New Vaccine Studies and CDC forced data release
1- People who recovered from COVID-19 and received a COVID-19 shot were more likely to suffer adverse reactions, according to a new study in Europe. The CEM study was rolled out in a period ranging from February 2021 to February 2023 across multiple European countries. A total of 29,837 participants completed at least the baseline and the first follow-up questionnaire for 1st and 2nd vaccination and 7,250 participants for the booster.
The percentage of participants who reported at least one Adverse Drug Reaction (ADR) is 74.32% (95%CI 73.82–74.81). Across all vaccine brands, people with prior COVID-19 (natural immunity) were 2.6 times as likely after dose one to suffer an adverse reaction, according to the new study. Keep in mind the study was just for minor reactions. Participants were only specifically asked to record a range of minor adverse reactions (ADRs)
2- Moderna's pipeline of mRNA vaccines in question of high adverse reactions according to a new article published in Science on March 1, 2024.
It is delaying the larger trials needed to show whether the concept works. In small safety and immune tests of the vaccine strategy, which relies on a series of messenger RNA (mRNA) shots, an unusually high percentage (20%) of recipients developed rashes, welts, or other skin irritations.
“We are taking this very seriously,” says Carl Dieffenbach, who heads the Division of AIDS at the National Institute of Allergy and Infectious Diseases, which funded a recent phase 1 trial of the vaccine. Researchers want to understand the cause of the skin problems and how to minimize them before expanding tests of the vaccines, which are made by Moderna. “We would be moving more quickly if this finding had not been observed,” says Mark Feinberg, who heads IAVI, a nonprofit that is the vaccine’s major sponsor.
3- Vaccinated patients had double the mortality risk. Vaccinated patients had a 70 percent risk of mortality compared with 37 percent in the unvaccinated group.
Among hospitalized patients with COVID-19, a new study found that vaccinated patients had a significantly higher risk of mortality. The February study published in Frontiers in Immunology found that mortality among vaccinated and unvaccinated patients was 70 percent and 37 percent, respectively, and that the overall survival rate was two times higher in the unvaccinated group.
4- COVID-19 vaccination may have contributed to an increase in deaths from neoplasms like cancer during the 2021–22 pandemic period, according to a recently published study. They investigated trends in death rates from neoplasms (ICD-10 codes C00-D48) for all age
groups in the US using data from the CDC (Centers for Disease Control and Prevention). Neoplasm refers to an abnormal mass of tissue caused by cells dividing and growing more than normal or not dying when they should.
The MC/UC cancer death rate ratio “tends to be relatively stable over time,” researchers wrote. While the ratio was “relatively stable” between 2010 and 2019, it jumped in 2020, and continued to rise in 2021 and 2022. As to the increase in MC/UC cancer death ratio seen in 2021 and 2022, “given the case studies of neoplasms following COVID-19 vaccination cited in the literature, one possible factor could be adverse effects of the COVID-19 vaccines.
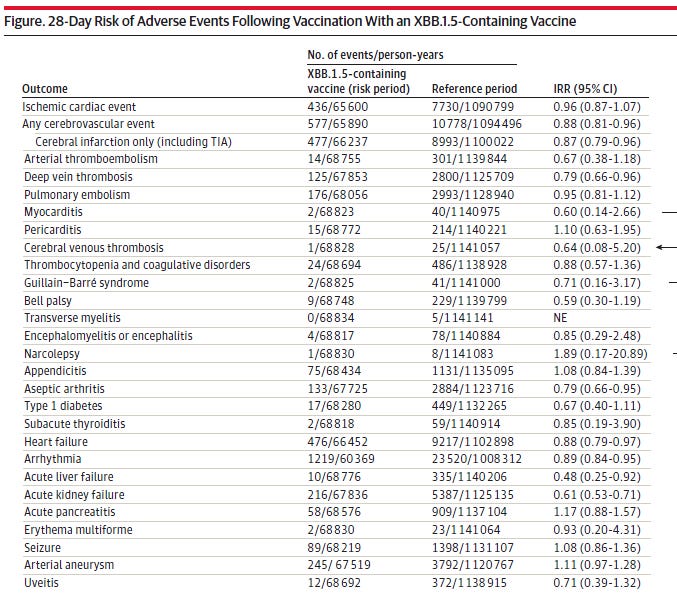
5- Denmark Study on 65 years plus receiving their 5th shot shows significant severe adverse events, but not much different than after the 4th shot. The study period was from Sept. 15, 2022 to Jan. 8, 2024 on just over 1 million people. The risk period was defined up to 28 days after the shot.
Comparing 5th shot (XBB1.5) to the reference period look at IRR (the difference) where 1.0 would be no difference. However, besides that, what is troublesome is the high number of overall adverse effects.
While any one event has less than 1% of occurring, which is high but these are old people and most with comorbidities. For 18 of the 28 adverse events examined, the upper bound of the CI was inconsistent with moderate to large increases in relative risk of 1.4 or greater.
For example the chance of Myocarditis is low but maybe you get Kidney failure instead. When I do a quick calculation, adding up all of these it comes to 4,417 person years of adverse effects over an average of around 68,000 person years. This means the odds are 6.5% or 6 and 1/2 of every 100 people will get one of these adverse events which many are lethal like heart or kidney failure among others. One could also get more than one of these problems. The one event with the highest odds is Arrhythmia at 1,219/60,369.
I don't think these shots are safe at any age and certainly not effective at any age.
The fact that court orders were required for Pfizer, Moderna, FDA and CDC to release Covid and Vaccine data is a huge red flag. Here is one of the latest outcomes.
The Centers for Disease Control and Prevention (CDC) tried to keep its V-Safe data hidden, but the U.S. health agency lost a legal battle with ICAN attorney Aaron Siri back in September of 2022.This decision mandated the CDC to provide 390,000 entries a month starting February 15, 2024, and continuing every month thereafter for the entire year.
Now with March 15th release, there is 780,000 free text submissions from the V-Safe vaccine safety monitoring system are available to the public. You can view all the data and there is just reams of it in a Microsoft spread sheet, but here are a few summaries from just the first batch of 390k. My comments in brackets
1 in 1,300 individuals reported experiencing Bell’s palsy (facial paralysis) in the initial few days after vaccination. ( A good friend of mine is neighbours with singer Justin Beiber and I am sure this is what he got. He cancelled his tour, sold his music rights and I doubt he will return).
1 in 906 individuals reported disturbances in their normal menstrual cycle following vaccination. ( have not asked any women about this?)
1 in 450 individuals reportedly experienced shingles after receiving the vaccine. (it was much higher in old age homes, I know a couple instances where over half in the home got shingles)
1 in 160 individuals reported tinnitus or ringing in the ears. ( I know a couple people mentioned this)
1 in 143 individuals experienced heart palpitations after vaccination during the initial reporting period. (I know at least 3 people had severe heart problems after a shot).
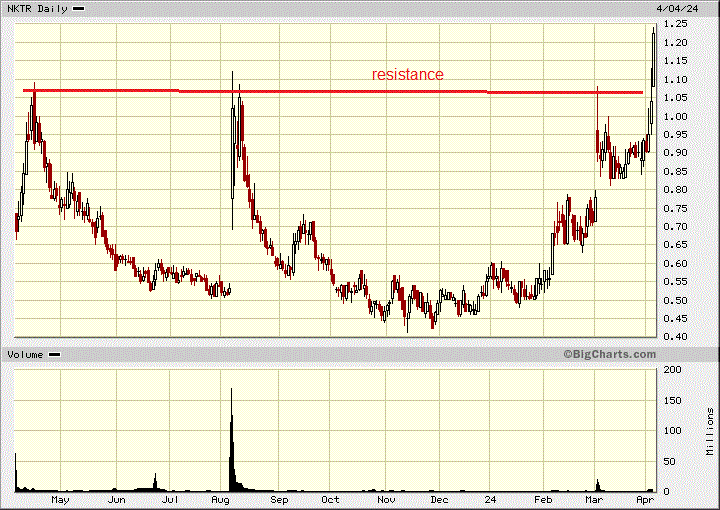
Now on a more positive note, Nektar broke out on the chart today.
Nektar - - - Nasdaq;NKTR - - - - Recent Price - $1.22
Entry Price - $0.68 - — - - - Opinion - hold
The stock is still only about half it's cash value and remember they recently did an above market financing at $1.30. I am sure that fund wants to make a profit above $1.30. I have a target of $1.60 to $2.00 on the stock.
All forecasts and recommendations are based on opinion. Markets change direction with consensus beliefs, which may change at any time and without notice. The author/publisher of this publication has taken every precaution to provide the most accurate information possible. The information & data were obtained from sources believed to be reliable, but because the information & data source are beyond the author's control, no representation or guarantee is made that it is complete or accurate. The reader accepts information on the condition that errors or omissions shall not be made the basis for any claim, demand or cause for action. Because of the ever-changing nature of information & statistics the author/publisher strongly encourages the reader to communicate directly with the company and/or with their personal investment adviser to obtain up to date information. Past results are not necessarily indicative of future results. Any statements non-factual in nature constitute only current opinions, which are subject to change. The author/publisher may or may not have a position in the securities and/or options relating thereto, & may make purchases and/or sales of these securities relating thereto from time to time in the open market or otherwise. Neither the information, nor opinions expressed, shall be construed as a solicitation to buy or sell any stock, futures or options contract mentioned herein. The author/publisher of this letter is not a qualified financial adviser & is not acting as such in this publication.